History and Structure
The history of this polymer is an interesting one.
Stephanie Kwolek was part of a group working for the American company DuPont that were looking for a new, lightweight fibre that was strong enough to be used in tires. She was working with two polymers in 1964 (poly-p-Phenylene-terephthalate and polybenzamide), which formed a cloudy opalescent liquid that had interesting properties of forming liquid crystals.
Initially they were just disposing of this substance, but one day Stephanie decided to get it tested in a machine called a spinneret, which is what is used to "pull" the fibres out of polymers. They found that unlike nylon and a lot of other polymers that they were used to, this one didn't break!
The polymer was officially discovered in 1965, and by 1971 Kevlar as we know it today was branded and becoming available.
As for the structure of this molecule, here's an image to help give you an idea:

The two two monomers 1,4-phenylene-diamine (para-phenylenediamine) and terephthaloyl chloride are synthesized through a condensation reaction, though we'll go more in depth about this in the "Production and History" page.
Here's an image of Kevlar, you'll see it repeating the pattern from the previous image:
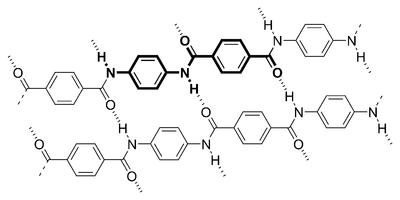
As you can see in the image, there are many hydrogen bonds (dashed lines) which contribute to Kevlar's extreme tensile strength. They form between the carbonyl groups on one monomer to the NH bonds on the other monomer.
Additionally, you should see that it is composed of what looks like hexagons, but in the chemistry world they are referred to as aromatic hydrocarbons. These hydrocarbons do what is called "pi stacking", which just means, in short, that they are attracted to other aromatic hydrocarbons. This repeating stacking contributes heavily to Kevlar's strength as well.
Check out our page Production and Recycling to learn more about this polymer!